
Program description
EP4 receptor antagonist
- Discovery
- Candidate
- Preclinical
- Phase I
DT-9081: a best-in-class EP4R antagonist overcoming PGE2-mediated immunosuppression
DT-9081, an EP4 receptor (EP4R) antagonist represents a breakthrough in cancer therapeutics. This orally administered small molecule is designed to reverse the immunosuppressive effects of Prostaglandin E2 ( PGE2 ) produced by the COX-2 positive tumors. By doing so, DT-9081 restores an immunocompetent microenvironment, driving tumor regression in specific cancer indications.
DT-9081 is being evaluated in the EPRAD study, an open-label Phase I trial conducted across multiple centers. This study focuses on patients with advanced solid tumors that have either recurred or metastasized. The trial aims to gather data on the drug’s safety, tolerability, pharmacokinetics, pharmacodynamics, and initial efficacy in patients who have not responded to standard treatments. For more details, please visit clinicaltrials.gov NCT05582850.
The European Patent Office granted a patent for our proprietary EP4 receptor antagonist series, including DT-9081, reinforcing its uniqueness and potential impact.
DT-9081: our translational research and precision science in action
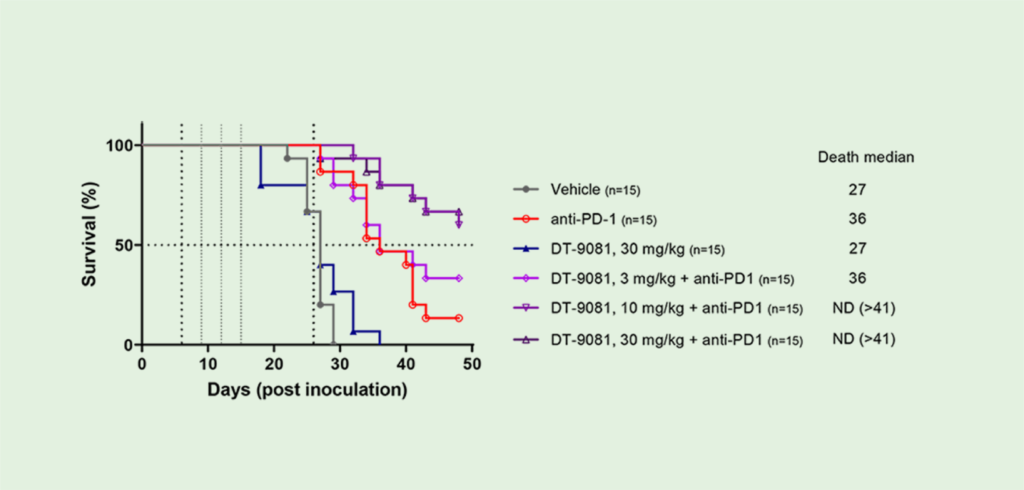
In pre-clinical studies, DT-9081 has shown significant antitumor effects in multiple models when combined with immune checkpoint inhibitors ( ICIs ) such as anti-PD1s. For instance, in a sarcoma model, DT-9081 significantly increased the response rate of an anti-PD1.
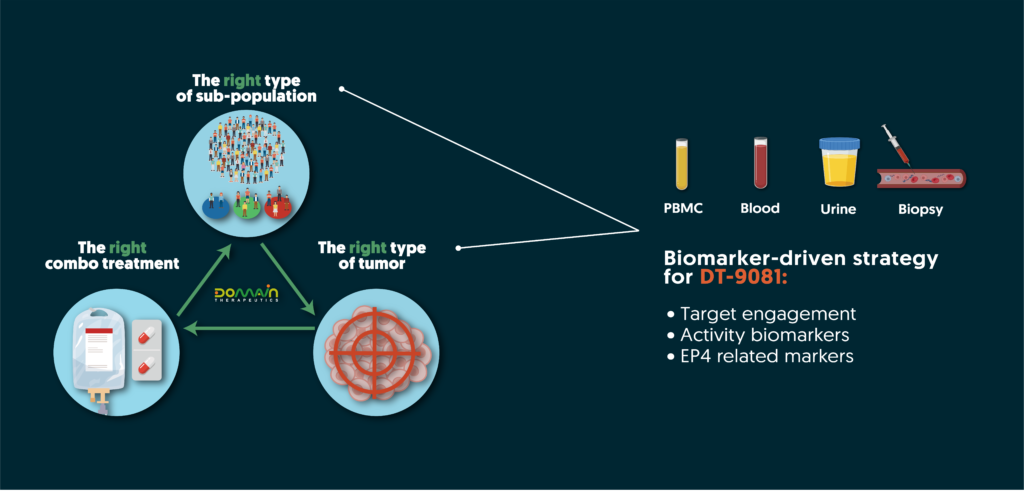
Leveraging comprehensive tumor profiling and in-depth GPCR pharmacology knowledge, our team has established a robust biomarker strategy. This strategy guides clinical positioning and monitoring of an EP4 receptor’s activity during treatment, de-risking clinical development and ensuring efficient clinical trials with highly precise and informative biomarkers. By identifying the right indication and the right sub-population of patients, Domain aims to improve clinical success and patient outcomes.
Our lead clinical candidate is also involved in the CONDOR project led by Prof. Antoine Italiano. Set to move into a Phase II trial in the first half of 2025, DT-9081 has the potential to improve immune responses in soft tissue sarcoma ( STS ) and address resistance mechanisms to current therapies.
Therapeutic benefits of DT-9081: driving the efficacy of cancer immunotherapy
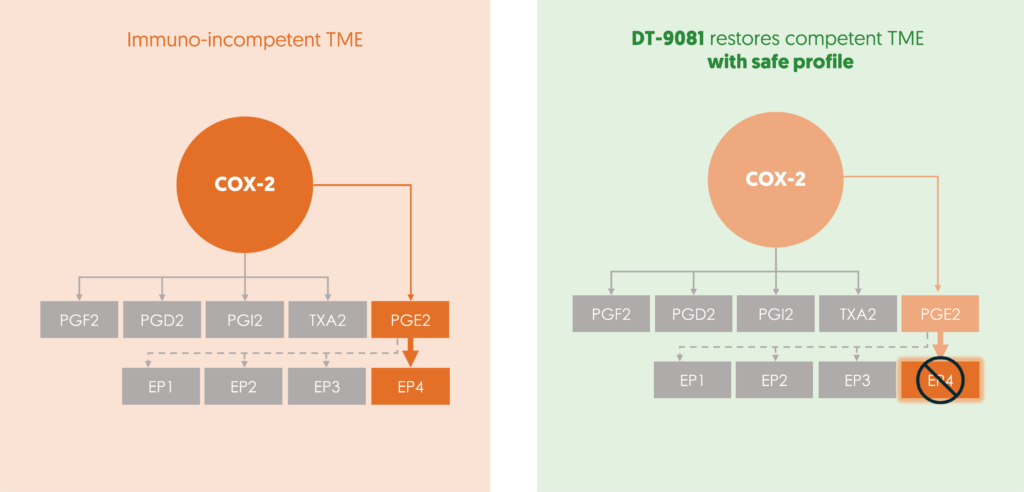
DT-9081’s precision targeting of the EP4 receptor disrupts PGE2-mediated immunosuppression within the tumor microenvironment. This restoration of the immune system efficacy enables recognition and the cancer cells to be attacked. Simultaneously, DT-9081 amplifies the activity of immunocompetent cells ( Dendritic, NK, CD8 ) leading to antitumor T cell immunity and inhibiting tumor proliferation and metastasis. With an excellent safety profile and a strong biomarker plan in place, DT-9081 holds immense promise in improving a patient’s response to immunotherapy.
Reversing GPCR-mediated immunosuppression to optimise anticancer treatments
Although several current antitumor interventions, including chemotherapy and certain ICIs, can be effective, immune resistance to these treatments is associated with a boost of PGE2 secretion by cancer cells, leading to immune evasion and tumor development, resulting in poor prognosis for patients.
Targeting the COX-2/PGE2/EP4 axis with selective EP4 receptor antagonism is a potent therapeutic strategy. This approach restores cancer recognition by the immune system, overcomes immunotherapy resistance and optimizes the efficacy of anticancer treatments.
Combining DT-9081 with anticancer therapies, with the aim of boosting the immune system, could lead to more sustainable and potent treatments, extending innovative treatment options for cancer patients.
Additional resources
• DT-9081 is a novel EP4 receptor antagonist that has a potential to inhibit tumor growth and enhance immune response in patients with advanced solid tumors.
• DT-9081 has demonstrated an acceptable safety profile in patients with advanced solid tumors, with no DLT reported at the highest dose levels tested (400 mg and 600 mg).
• DT-9081 PK exposure increases in a dose-proportional manner and the dose of 600 mg demonstrated the best target coverage profile.
• DT-9081 was able to induce a sustained EP4 target engagement by means of cytokine release measurements.
• The collective evaluation of the safety and tolerability, PK and PD profile of DT-9081 allowed to select 600 mg as the Recommended Phase 2 Dose [RP2D) for further clinical development in solid tumors.
This poster presents the complete characterization of Domain drug candidate benchmarked to EP4 antagonist molecules currently in the clinic and demonstrating a best-in-class potential.
Phase Ia clinical candidate DT-9081, a selective EP4 receptor antagonist, was designed to counteract the PGE2-immunosuppressive effects in the TME and to synergize with ICI. DT-9081 in vivo efficacy, in combination with ICI in murine syngeneic models, is presented.
DT-9081 is a new small molecule, orally administered, highly selective antagonist of EP4R developed to overcome the immune-suppressive effects of PGE2, and to reverse the resistance to immune checkpoint blockers.
DT-9081 has demonstrated significant anti–tumor activity as monotherapy and in combination with immunotherapeutic agents in several syngenic murine cancer models (CT26 colorectal tumor model and MCA205 sarcoma model).